In claiming that developmental processes are too integrated to allow change, Explore Evolution ignores over 10 years of research in evo-devo on the modularity of development. Evolutionary developmental biologists who study Hox genes think that mutations in the CREs of target genes for Hox genes are more likely to be more important for morphological evolution than mutations in Hox genes themselves.
Developmental biologists are investigating another kind of mutation - mutations in "hox genes" - that many neo-Darwinists think can provide a signficant source of major variation in living forms. Hox genes are "master regulator" genes that turn other genes in the cell on and off during the developmental process Because hox genes are so important for coordinating the activities of the cell, some researchers think that mutations in these genes can cause large-scale changes in the structure of an organism.Explore Evolution, p. 109
Explore Evolution disregards research on Hox genes that strongly suggest that instead of soley acting at the top of a hierarchy as a "master regulator", they work at many sites in a developmental pathway as "micromanagers".
We still have little idea how the differential expression of one 'master' gene can control the morphology of complex structures, but recent studies suggest that the Drosophila Hox gene Ultrabithorax micromanages segment development by manipulating a large number of different targets at many developmental stages.Michael Akam (1998) "Hox genes: from master genes to micromanagers," Current Biology 8:676
Explore Evolution gestures towards accuracy in suggesting that there is a challenge in mutating the protein-coding regions of Hox genes. As Sean Carroll and colleagues explain below, developmental regulatory genes, such as Hox genes are pleitropic - they control many different developmental processes, making it more difficult to mutate their protein-coding region without harmful consequences (although the case of the evolution of Ultrabithorax function in limb repression in insects appears to be a notable exception). In contrast to most mutations in the protein-coding regions of developmental regulatory genes, mutations in CRE's minimize the fitness penalty - an important issue which is completely ignored by Explore Evolution.
A clear principle is emerging from the increasing number of case studies: pleiotropy imposes a genetic constraint on the type of changes that can be accommodated in morphological evolution. Highly pleiotropic genes (including most developmentally regulated genes) are more likely to contribute to morphological evolution through cis-regulatory changes than through coding sequence alterations. In contrast, known examples of pigmentation evolution resulting from the alteration of coding sequences affect genes involved in a single process, such as the overall body color in fish, mammals, or birds. Coding sequence changes appear to be better tolerated in minimally pleiotropic genes. This principle of minimizing fitness penalties delimits the scope of what changes are permissible under natural selection and explains why CRE evolution is a pervasive mechanism underlying morphological diversification.Prud'homme et al., (2007) "Emergining Principles of Regulatory Evolution," PNAS 104:8609
To understand how Hox genes, such as Ultrabithorax, act to regulate developmental pathways, we must consider how CREs act as genetic switches. Recall that the four-winged fruit fly is generated by mutations in Ultrabithorax, (Ubx) that turn the gene off in hindwings (halteres).
 A Four-Winged Fly
A Four-Winged Fly The diagram below shows the basic gene network involved in patterning the forewing along the anterior-posterior and dorsal-ventral axes. If Ubx was working as a "master regulator" in the hindwing, Ubx would be predicted to only affect genes at the top of the hierachy, such as selector or short-range signals. Instead, it has been shown by Sean Carroll and colleagues (Weatherbee et al., 1998, Hersh et. al, 2005) that Ubx may directly regulate many wing patterning genes at all levels of the hierarchy, including the primary target genes, demonstrating that Ubx is acting as a micromanager.
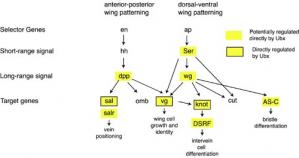 Wing Patterning
Wing Patterning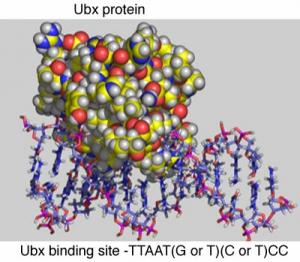 Ubx 10
Ubx 10 How can Ubx regulate multiple genes involved in wing patterning? Ubx binds to CREs that contain the specific DNA sequence as shown in the diagram below.
This short sequence is present in many different CREs throughout the genome. CREs also have different binding sites for other proteins that can turn genes on or off. In the figure below, future wing cells lack the Ultrabithorax protein, Ubx (U), while future haltere cells contain Ubx. Additionally, there are other proteins (A,B,C,D,E,F) which bind to the CRE to regulate the nearby target gene.
Note that whether or not Ubx can bind to a CRE depends upon whether Ubx is present and if the CRE has a binding site on it for Ubx. For example, Omb does not have a Ubx binding site and is not regulated by Ubx. In contrast, sal and CG13222 have Ubx binding sites and are respectively turned off or on in halteres.
Is there direct evidence that Ultrabithorax binds to CREs of target genes involved in wing patterning? So far Sean Carroll's lab has identified four such genes (Galant et al., 2002; Hersh and Carroll, 2005; and Hersh et al, 2007).
Since the proteins which bind to CREs generally bind to different sites on the CRE, a mutation in a CRE which abolished the binding of Ubx would not affect the binding of A,B,C etc. to their sites on the CRE. This independence of binding sites on CREs means that these binding sites can evolve independently.
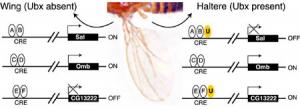 Ubx and CRE
Ubx and CRE As we have seen with four-winged fruit flies, it requires a great many coordinated changes to transform one system into another without losing function in the "in-between" steps. The more the individual parts of a system depend on each other, the harder it is to change any one part without destroying the function of the organism as a whole. Since Hox genes affect so many genes and systems, it seems unlikely that they could be mutated without damaging the way some of the genes are switched 'on or 'off.'Explore Evolution, p. 109
How is a hindwing transformed into a forewing? In this case, "a great many coordinated changes" are actually mutations in three CREs controlling in which part of the hindwing the Hox gene Ultrabithrox is normally expressed. These three mutations result in the absence of Ultrabithorax in all of hindwing and the conversion of a hindwing into a forewing. As we shall see, mutations of CREs, even in genes that are involved in highly complex networks, such as Hox genes, are very capable of evolving.
Can protein-coding regions of Hox genes be changed? Elsewhere, we examine the case of how Ultrabithorax evolved the ability to repress limbs in insects by the replacement of the amino acids serine or threonine with the amino acid alanine at the tail of Ultrabithorax. In that example, "a great many changes" may involve as much as five such replacements, hardly an insurmountable barrier.
